-
Altman RD, Moskowitz R; HYALGAN® Study Group. Intraarticular sodium hyaluronate (HYALGAN®) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. J Rheumatol. 1998;25(11):2203-2212.
-
Hammesfahr JFR, Knopf AB, Stitik T. Safety of intra-articular hyaluronates for pain associated with osteoarthritis of the knee. Am J Orthop. 2003;32(6):277-283.
-
Kelly MA, Goldberg VM, Healy WL, Pagnano MW, Hamburger MI. Osteoarthritis and beyond: a consensus on the past, present, and future of hyaluronans in orthopedics. Orthopedics. 2003;26(10):1064-1079.
-
HYALGAN®, data on file. Fidia Farmaceutici S.p.A., Italy.
-
HYALGAN® (sodium hyaluronate), approval letter. Food and Drug Administration Web site. http://www.accessdata.fda.gov/cdrh_docs/pdf/P950027a.pdf. Accessed August 18, 2011.
-
Carrabba M, Paresce E, Angelini M, Re KA, Torchiana EEM, Perbellini A. The safety and efficacy of different dose schedules of hyaluronic acid in the treatment of painful osteoarthritis of the knee with joint effusion. Eur J Rheumatol Inflamm. 1995;15(1):25-31.
-
Bragantini A, Cassini M, De Bastiani G, Perbellini A. Controlled single-blind trial of intra-articularly injected hyaluronic acid (HYALGAN®) in osteo-arthritis of the knee. Clin Trials J. 1987;24(4):333-340.
-
Grecomoro G, Martorana U, Di Marco C. Intra-articular treatment with sodium hyaluronate in gonarthrosis: a controlled clinical trial versus placebo. Pharmatherapeutica. 1987;5(2):137-141.
-
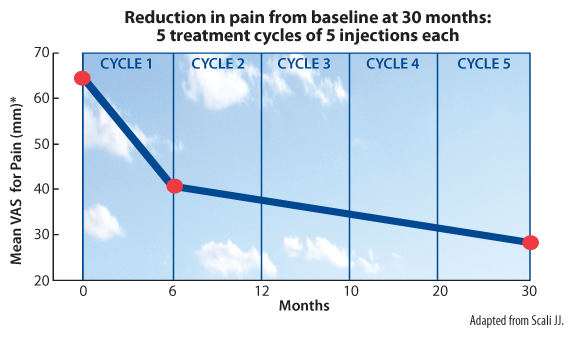
Scali JJ. Intra-articular hyaluronic acid in the treatment of osteoarthritis of the knee: a long term study. Eur J Rheumatol Inflamm. 1995;15(1):57-62.
-
Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular-weight dependent? Semin Arthritis Rheum. 2002;32(1):10-37.
-
Balazs E. The physical properties of synovial fluid and the specific role of hyaluronic acid. In: Helfet AJ, ed. Disorders of the Knee. 2nd ed. Philadelphia, PA: JB Lippincott; 1982:61-74.
-
Gotoh S, Miyazaki K, Onaya J, Sakamoto T, Tokuyasu T, Namiki O. Experimental knee pain model in rats and analgesic effect of sodium hyaluronate (SPH) [in Japanese]. Nippon Yakurigaku Zasshi. 1988;92(1):17-27.
-
Pozo MA, Balazs EA, Belmonte C. Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Exp Brain Res. 1997;116:3-9.
-
HYALGAN®. [package insert]. Abano Terme, Italy: Fidia Farmaceutici; May 2014
-
Neustadt DH. Long-term efficacy and safety of intra-articular sodium hyaluronate (HYALGAN®) in patients with osteoarthritis of the knee. Clin Exp Rheumatol. 2003;21(3):307-311.
-
Stitik TP et al. Arch Phys Med Rehabil. 2007;88(2):135-141
-
Turajane T, Labpiboonpong V, Maungsiri S. Cost analysis of intraarticular sodium hyaluronate treatment in knee osteoarthritis patients who failed conservative treatment. J Med Assoc Thai. 2007;90(9):1839-1844.
-
Parmet S, Lynm C, Glass RM. Osteoarthritis of the knee. JAMA. 2003;289(8):1068.